Consider the Following Which Structures Can Exist as Cis-trans Isomers
Alkenes of the type RCHCHR can exist as cis and trans isomers. Alkenes with a CCR 2 unit where the two R groups are the same do not exist as cis-trans isomers.
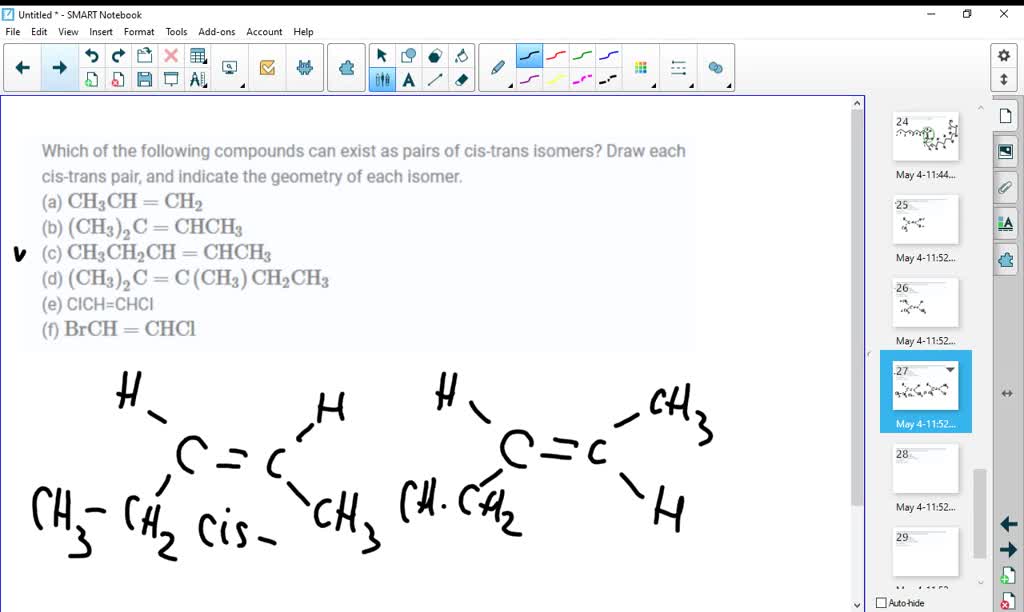
Solved Which Of The Following Compounds Can Exist As Pairs Of Cis Trans Isomers Draw Each Cis Trans Pair And Indicate The Geometry Of Each Isomer A Mathrm Ch 3 Mathrm Ch Mathrm Ch 2 B Left Mathrm Ch 3 Right 2 Mathrm C Mathrm
The boiling point of a molecule depends upon dipole-dipole interactions.

. All of these choices are constitutional isomers. All four structures have a double bond and thus meet rule 1 for cis-trans isomerism. 5 Points CH3CH2CH2CHCHCH2CH2CH3 CH3CH2CH2CH2CH2CH2CHCH2 CH3CH2CHCHCH2CH2CH2CH3 CH2CHCH2CH2CH2CH2CH2CH3 III IV O II and III O I and II O I alone O I and IV O I and III.
Non-Stereoisomeric Alkenes One important thing to emphasize about the identical groups. CH 3 2 CCHCH 2 CH 3. Consider the alkene with the condensed structural formula CH 3 CHCHCH 3.
All four structures have a double bond and thus meet rule 1 for cis-trans isomerism. Cis-trans isomerism can arise in carbon chemistry from the presence of i an olefinic bond C C or ii the presence of a ring structure. Consider the following.
The first asks you if this each of these following compounds our system trans or are able to form system Transit Summers and if so you want to draw them out. The following substituents are attached to a cyclohexane ring. Which of the following can exist as cis-trans isomers.
Tap card to see definition. 14 Consider the following. This compound meets rule 2.
The isomer in which the two chlorine Cl atoms lie on the same side of the molecule is called the cis isomer Latin cis. The former is solid at room temperature melting point 43 o C and the latter is found to be liquid with a melting point of 134 o. It exists as both cis and trans isomers.
Which structures can exist as cis-trans. Which of the following species isare not a resonance forms of the species in the box. We could name it 2-butene but there are actually two such compounds.
So when youre thinking about CIS and trans I Summers what you want to look at is you want to look at the two carbons on the double bond and you want to make sure that these two carbons. Answer a 1 and 3 can exits as cis-trans isomers. Which structures can exist as cis-trans.
CH 3 CHCHCH 2 CH 3. Since cis-isomer has a higher dipole moment therefore it has a higher boiling point. CH 3 2 CCHCH 2 CH 3.
To summarize as long as there are two identical groups atoms on both carbons of the double bond the alkene can be classified as cis or trans. The double bond results in cis-trans isomerism Figure 133 Ball-and-Spring Models of a Cis-2-Butene and b Trans-2. I missed that oneCan u please point out the rest that ive got wrong.
It exists as both cis and trans isomers. Name each of the. 2 and 4 cannot exist as cis and trans isomers because identical substituents are bonded to.
Ill draw them outYoull save me a lot of timeCuz we also have to draw each isomer. This compound meets rule 2. Cyclohexane methylcyclohexane 11-dimethylcyclohexane 12-dimethylcyclohexane both c and d all of the above ANS.
Take an olefin with an internal double bond say 2-butene H 3C CH CH CH 3. 1 from which it can be readily inferred that their ability to assemble into supramolecular structures fit in subcellular struc- tures or interact with enzymes and other molecules is different. Trans the two hydrogen atoms are on opposite sides cis the two hydrogen atoms are on the same side as are the two ethyl groups cis the two ethyl groups are on the same side neither fliping the bond does not change the molecule.
Benzene exist as two equally contributing resonance structures-The bond lengths are an average of a single bond and a double bond with the electrons moving in a circle. The term aromatic is a structural term that applies to cyclic conjugated molecules that are planar and lack alkene like reactivity due to enhanced resonance stabilization. Draw the structures of cis- and trans-isomers of the following compounds.
This sort of isomerism can also arise in inorganic chemistry also but I will confine the discussion to organic examples. Cis if the two R groups are on the same side of the carbon-to-carbon double bond and trans if the two R groups are on opposite sides of the carbon-to-carbon double bond. Thus the all-E-isomers of carotenoids are linear and rigid molecules whilst the cis isomers have a kinked shape Fig.
Draw 7 constitutional isomers of a cycloalkane with the formula C6H12. Chemistry questions and answers. This is known as the trans isomer.
The structures of cis- and trans-isomer of hex-2-ene are. Elaidic acid and oleic acid are cis-trans isomers. Alkenes and cyclic compounds can exhibit cis-trans isomerism.
For those compounds that can exist as cis and trans isomers draw and label the isomers. View the full answer. CH 2 CBrCH 3.
The difference between the two is that the 1 Cis Isomer is polar whereas the Trans Isomer is non-polar. 14 Which of the following structures can exist as cis-trans isomers. Which structures can exist as cis-trans isomers.
Which of the following CAN exist as cis-trans isomers. Click card to see definition. Maleic acid is the cis isomer and fumaric acid is the trans isomer.
In 12-dichloroethene Table 1 however restricted rotation about the double bond means that the relative positions of substituent groups above or below the double bond become significantThis leads to a special kind of isomerism. CH 3 CHCHCH 2 CH 3. So there is cistrans isomerism with respect to HOh yes.
СНCHCH-СНCH-CHCHCH A I and II B I and IV E I alone C I and III D II and III. Note that cis and trans isomers are different compounds with distinct physical and chemical properties. It has two nonidentical groups on each carbon atom H and Cl on one and H and Br on the other.
CH2CH2CH2CH-CHCH2CH2CH3 CH3CH2CH2CH2CH2CH2CHCH2 I II CH3CH2CHCHCH2CH2CH2CH3 CH2CHCH2CH2CH2CH2CH2CH3 III IV Which structures can exist as cis-trans isomers. The double bond results in cis-trans isomerism Figure 133 Ball-and-Spring Models of a Cis-2-Butene and b Trans-2-Butene. Consider the case of 12-dichloroethene In one the two chlorine atoms are locked on opposite sides of the double bond.
A I and II B. Cyclohexane methylcyclopentane 12-dimethylcyclobutane 11-dimethylcyclobutane 13-dimethylcyclobutane 123-trimethylcyclopropane and 112-. 2Cis isomer due to being polar shows Dipole moment while the trans isomer does not.
CH 2 CBrCH 3. It has two nonidentical groups on each carbon atom H and Cl on one and H and Br on the other. On the first C there is H CH 3 on the second C there is H CH 2 CH 2 CH 3.
Consider the alkene with the condensed structural formula CH 3 CHCHCH 3. We could name it 2-butene but there are actually two such compounds.
What Is The The Structure Of The Cis Trans Isomers Of 2 Pentene Quora
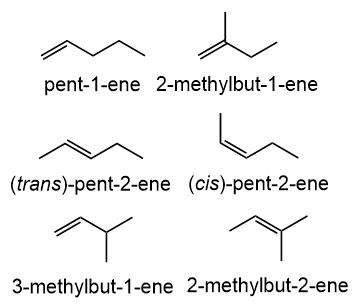
Including Cis Trans Isomers How Many Different Alkenes Have The Formula C5h10 Study Com
Are Cis 2 Hexene And Trans 3 Hexene Stereoisomers Quora
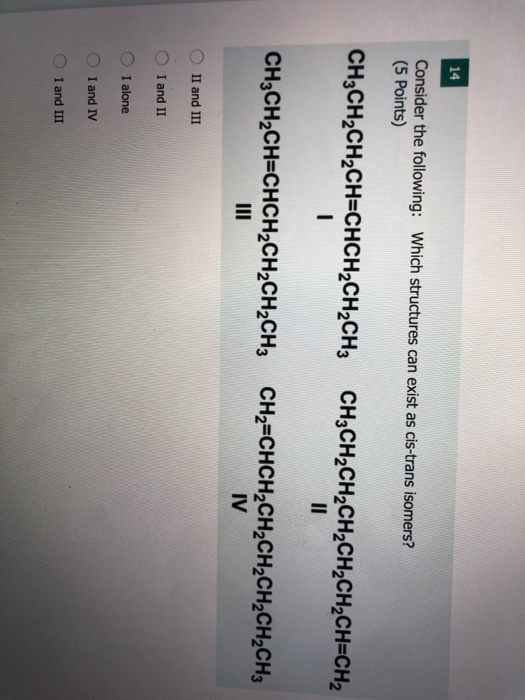
Solved 14 Consider The Following Which Structures Can Exist Chegg Com
Which Of The Following Best Describes Cis Trans Isomers And Why Quora
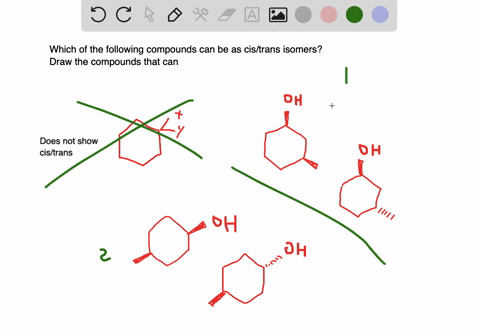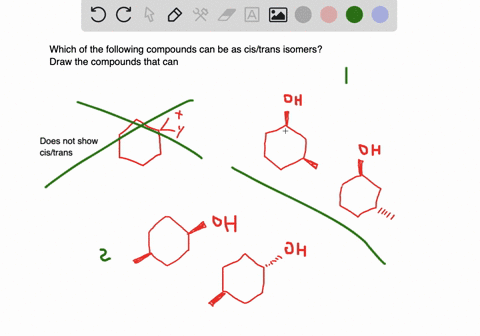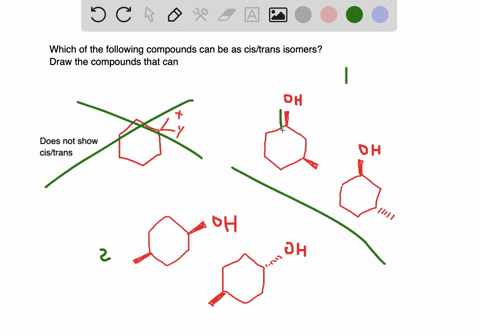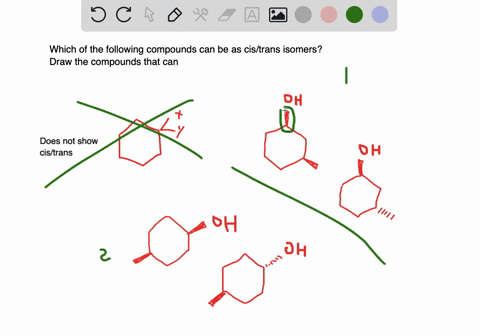
Solved A Which Of The Following Compounds Can Exist As Cis Trans Isomers B For Those Compounds That Can Exist As Cis And Trans Isomers Draw And Label The Isomers
Solved Draw The Structures Of The Cis Trans Isomers For Each Compound Course Hero
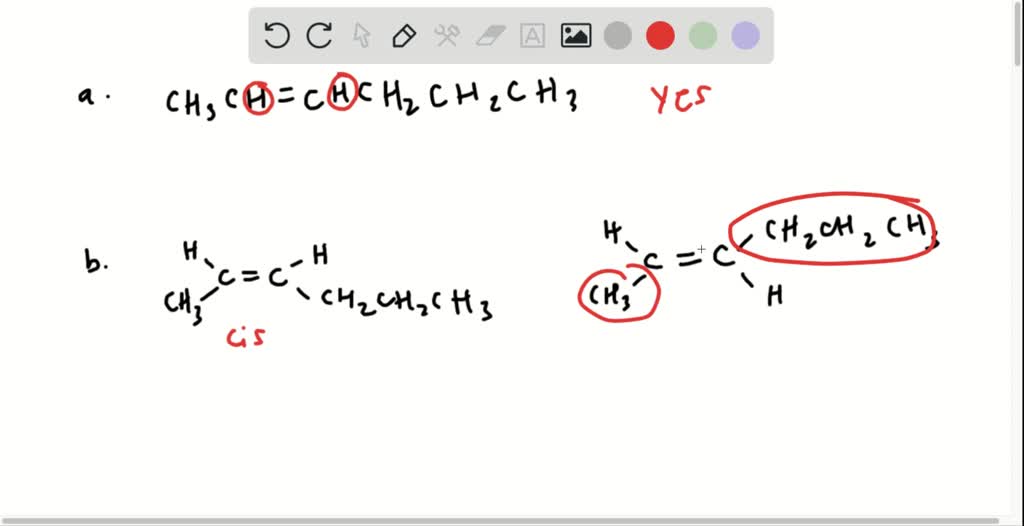
Solved A Which Of The Following Compounds Can Exist As Cis Trans Isomers B For Those Compounds That Can Exist As Cis And Trans Isomers Draw And Label The Isomers
How Is The Structure Of Cis Trans Isomer 4 Methyl 2 Pentene Supposed To Look Like Quora
Which Of The Following Best Describes Cis Trans Isomers And Why Quora
Does 2 Methyl 2 Butene Have Cis Trans Isomers Quora

Chemical Structures Of The Two Enantiomers Both For Cis And Download Scientific Diagram
How Is The Structure Of Cis Trans Isomer 4 Methyl 2 Pentene Supposed To Look Like Quora
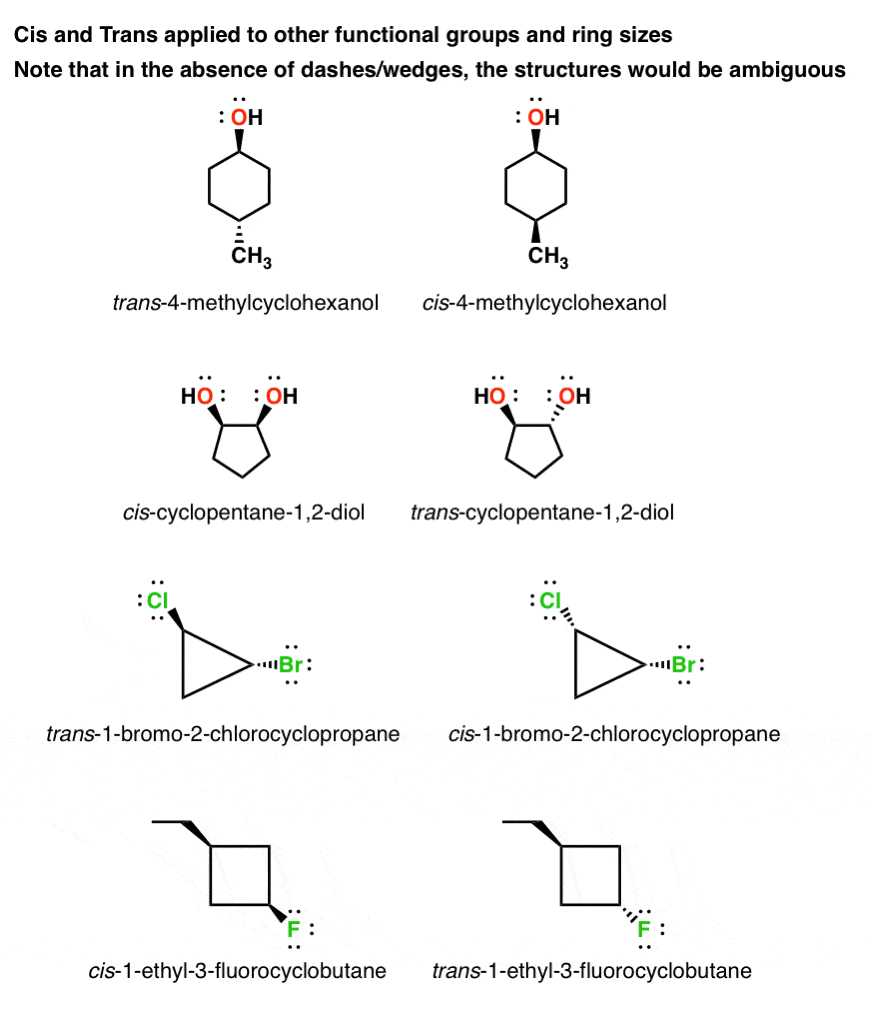
Geometric Isomers In Small Rings Cis And Trans Cycloalkanes
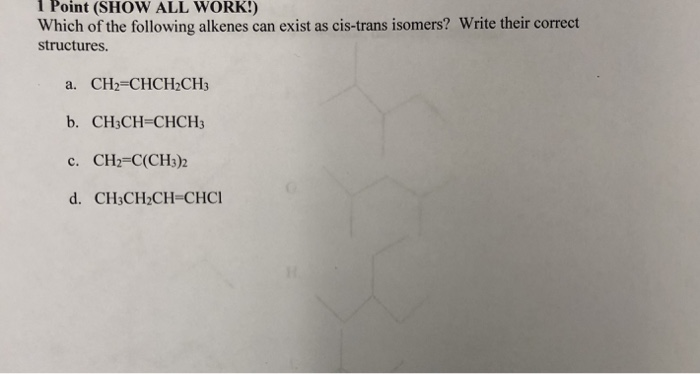
Solved L Point Show All Work Which Of The Following Chegg Com

Draw Cis And Trans Isomers Of The Following Compounds Also Write Their Iupac Names I Chci Chci Ii C2h5cch3 Cch3c2h5
What S The Difference Between Cis Trans Isomers And Stereoisomers Quora

Cis And Trans Isomers And Cis Trans Practice Problems Chemistry Steps
Comments
Post a Comment